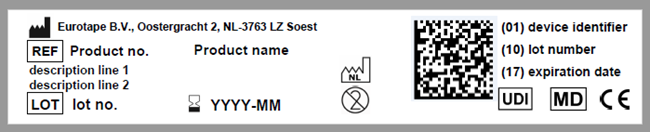
As a manufacturing company of medical devices, it is crucial to continuously comply with the 𝐌𝐞𝐝𝐢𝐜𝐚𝐥 𝐃𝐞𝐯𝐢𝐜𝐞 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐢𝐨𝐧 (MDR). Therefore we started labeling all our CE-marked products with a product label containing a UDI code. The UDI includes:
✅ 𝐀 𝐝𝐞𝐯𝐢𝐜𝐞 𝐢𝐝𝐞𝐧𝐭𝐢𝐟𝐢𝐞𝐫 (𝐔𝐃𝐈-𝐃𝐈)
✅ 𝐀 𝐩𝐫𝐨𝐝𝐮𝐜𝐭𝐢𝐨𝐧 𝐢𝐝𝐞𝐧𝐭𝐢𝐟𝐢𝐞𝐫 (𝐔𝐃𝐈-𝐏𝐈)
The UDI system improves the traceability, it enhances the safety of devices once they are on the market and supports monitoring by the relevant authorities. It also helps prevent medical errors and combats counterfeit devices.